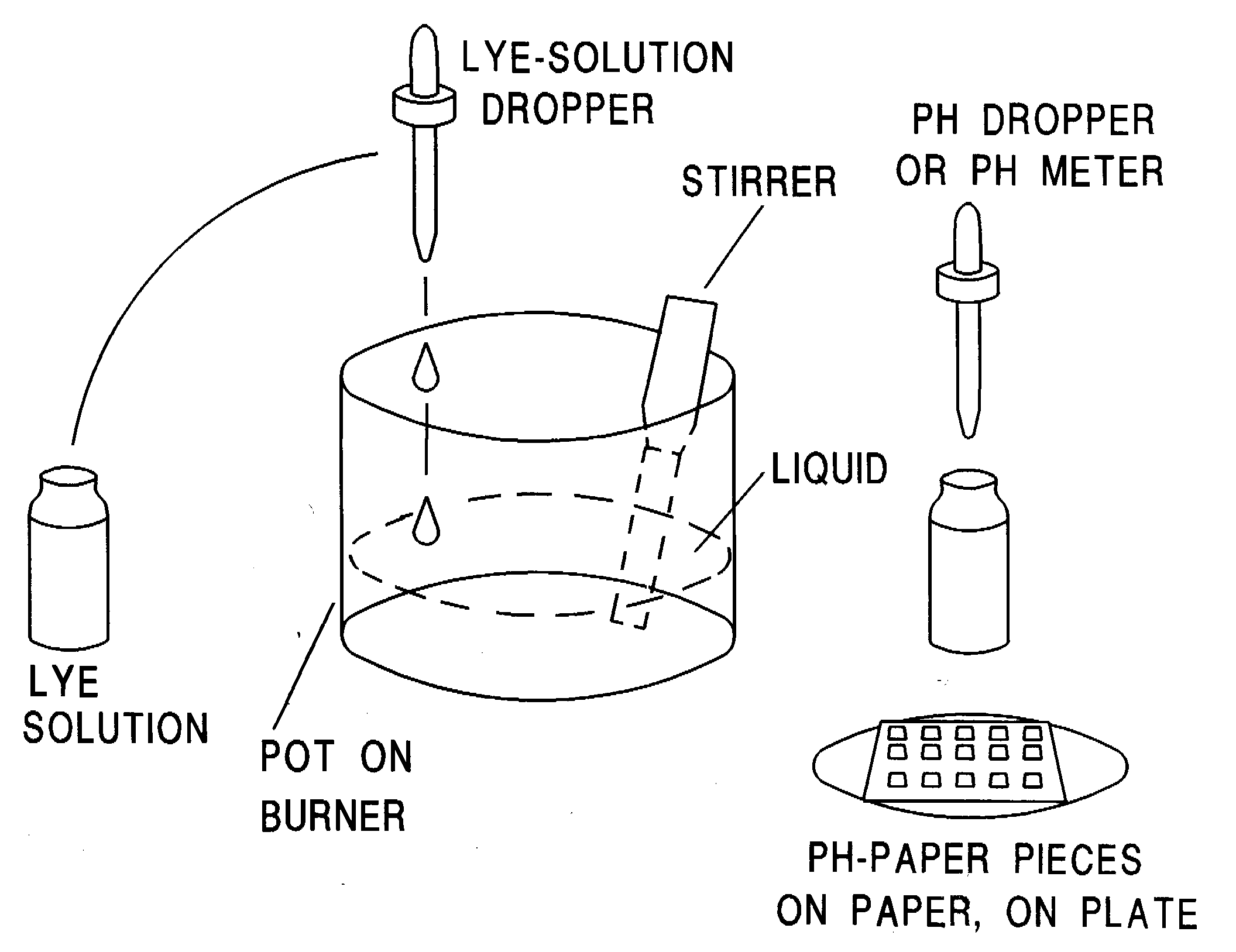
First you need to prepare a dilute lye solution. Label an eyedropper bottle or squirt bottle "Lye-poison" so the bottle will not confused with something else. Work in a sink so that any spills will be contained. Lye gives off eye-stinging fumes when mixed with water. To avoid inhaling fumes, hold your breath and wear goggles while doing the following procedure.
Working over a sink, put 8 teaspoons of distilled water in a sturdy glass then stir in 1 teaspoon of lye. Stir until the lye is dissolved. Heat will be generated as the lye dissolves and the glass may get fairly hot. You may want to close your eyes to avoid eye-stinging fumes, taking a peek periodically.
Pour the lye solution into a labeled eyedropper bottle or squirt bottle.
If you are using pH paper, tear off several 1/4" pieces and put them on a piece of white paper on a plate.

For the best accuracy, recalibrate the pH paper throughout the day with changes in temperature and humidity, as well as day-to-day. Buffer solutions of pH 4, 7 and 10 will help with this. Sources of pH buffer solutions are listed near the end of this document under LAB SUPPLIES.
If you are using dried sea minerals, mix 1/2 cup of dry material with 2 cups of distilled water. This makes sea water. Now proceed as described below:
2. If the starting material does not contain magnesium hydroxide (sea water does contain magnesium hydroxide), add some, or add a teaspoon of Epsom salts per gallon of water.
3. Pour the sea water into a stainless steel pot. Slowly, drop-by-drop, add the lye solution WHILE STIRRING. Every ten drops or so, test the pH. You might want to take at least 3 to 5 samples from different regions of the liquid. If you are using pH paper, the goal is to bring the pH up to 9.5, then stop to be on the safe side. If you are using a pH meter, stop just before you get to pH 10.78.
A white precipitate which includes m-state elements will form.
CAUTION: You must proceed slowly and patiently so that you do not exceed pH 10.78 with a meter or pH 9.5 with pH paper. If you go higher than pH 10.78, you might get a "Gilcrest precipitate" of toxic heavy metals. It is alleged that the Dead Sea salt water does not produce any Gilcrest precipitate. This has not been proven and should not be assumed.
4. Once you are at the correct pH, stop.
5. Pour the solution into a clean glass jar or test tube.
6. The white precipitate (slurry) slowly settles on the bottom of the jar. Let the slurry settle overnight. If metals or other toxins have been ruled out by prior testing of your starting material, the slurry is probably mostly calcium hydroxide, Mg(OH)2, lye, and a small amount of m-state.
You can speed this settling process with a centrifuge, which forces the precipitate to settle rapidly. Inexpensive second-hand centrifuges may be found at American Science and Surplus, http://www.sciplus.com.
7. Using a large syringe (or siphon), remove the liquid above the slurry.
8. Add distilled water to the precipitate (filling the jar), stir thoroughly, and let it settle again for at least 4 to 5 hours, preferably overnight.
9. Repeat steps 7 and 8 at least three times to thoroughly wash the precipitate. This should remove almost all of the lye. The remaining lye can be neutralized with HCl or distilled white vinegar as well. Washing three times is intended to reduce the dissolved "impurities" (like salt, for example) by 87.5%. Four washes would provide a 93.75% reduction, five washes a 96.875% reduction, and so on.
At this point, the precipitate is likely to contain some m-state, milk of magnesia Mg(OH)2, calcium, and perhaps some impurities.
Pour the precipitate and water into a stainless steel pot on a stove burner. A gas burner is preferred over electric because any magnetic fields from the electric burner may drive off some of the m-state material. Cover the pot with a lid to contain the m-state, and boil the solution for 5 minutes to sterilize it. Be careful not to spill the hot solution! Let it cool back to room temperature and recheck the pH to make sure it hasn't exceeded pH 9.
1. Boil before adding lye solution.
PROS: Faster reaction, faster precipitation. CONS: You may spill the hot lye solution. You may inhale fumes.
2. Boil while adding lye solution.
PROS: Faster reaction, faster precipitation. CONS: You may spill the hot lye solution. You may inhale fumes. Danger of lye spurting out of pot. Not recommended.
3. Boil and cool after adding lye solution.
PROS: No danger of inhaling fumes. Little danger of spilling hot lye solution. CONS: Slower reaction, slower precipitation.
4. Boil the washed precipitate (recommended).
PROS: No danger of inhaling fumes. No danger of spilling hot lye solution. pH is unlikely to change after boiling because the reaction has already taken place. CONS: Slower reaction, slower precipitation. If safety is the main issue, this seems to be the best method.
Caution: If you boil the solution on an electric burner, the magnetic field in the burner may "blow off" some of the m-state materials, resulting in a small yield. This can be minimized by adding a source of sodium (such as sodium hydroxide or salt) to the solution before boiling.
Since sea water contains sodium in salt, none of the boiling methods will be a problem with sea water. However, if you are starting with low-sodium fresh water, add a sodium source (such as table salt or lye solution) before boiling.
Once the precipitate and water have been sterilized, the next step is required to concentrate the m-state.
Here are four methods to separate Mg(OH)2 from m-state:
2. Use a syringe to remove the liquid over the precipitate, and discard the liquid. This leaves only the m-state/Mg(OH)2 precipitate.
3. To the wet precipitate, add hydrochloric acid (HCl) until you reduce the pH to 1.0 - 3.5. You can use muriatic acid (31% HCl) from a hardware store, but lab-grade HCl is less likely to be contaminated. A safe alternative to HCl is distilled white vinegar.
4. The white colloidal precipitate should dissolve, leaving a clear solution.
5. Add lye solution VERY SLOWLY drop-by-drop to bring the pH back up to 8.5 - 8.7. The precipitate that forms should be m-state mostly free of Mg(OH)2 (because m-state precipitates in this pH range, and Mg(OH)2 does not precipitate until pH 9.)
Note that your total yield may be diminished because you are not going past pH 8.7.
6. Remove the liquid above the precipitate, and wash the precipitate. It should be mostly m-state.
1. Dry the precipitate in a dark oven at about 275 degrees F for one or two hours. This forms a dry powder.
2. Take the dry powder and pulverize out any clumps.
3. In a glass container, cover the powder with some distilled water. For example, one liter of water for one cup of powder.
4. Add HCl or distilled white vinegar drop-by-drop to bring the pH to 5 or 6.
5. Shake the bottle and let it sit overnight. The dried m-state should not dissolve at that pH, but the Mg(OH)2 should dissolve.
6. The next day, after all the Mg(OH)2 has dissolved, pour everything into filter paper.
7. Wash the powder collected in the filter paper several times with distilled water to remove any residual traces of HCl or vinegar.
8. The washed powder may be oven-dried again at about 275 degrees F, and you should have m-state powder free of Mg(OH)2.
2. Mix the resulting powder with distilled white vinegar or 30% HCl. Everything which does not dissolve in m-state. This will be quite a small amount if you start with sea water. (If you mix pure HCl with distilled water, remember: ADD ACID TO WATER, NEVER ADD WATER TO ACID).
3. Measure the amount of HCl/m-state solution (or vinegar/m-state solution).
4. Add distilled water to the HCl/m-state solution. Add an amount of water that is at least ten times the amount of HCl/m-state solution. (You may substitute distilled white vinegar for HCl).
5. Filter the solution through 5 layers of coffee filters.
6. Wash the powder at least three times in a large amount of distilled water.
2. Filter out the precipitate.
3. To the remaining liquid containing only m-state, add HCl or distilled white vinegar drop-by-drop until the pH reaches 8.5.
4. Add lye solution drop-by-drop to bring the pH back up to 10.78. The resulting precipitate should be only m-state.
5. Wash the precipitate as described earlier.
6. To be safe, check the pH of the precipitate slurry. It should
be
9 or less before ingesting.
This method takes longer than the WET method. In some cases, it involves boiling lye for several hours, which may spray some caustic solution around your work area. Please wear neoprene gloves, a PVC lab apron, and eye goggles when you use this method. Sources for this safety clothing are listed near the end of this document under LAB SUPPLIES.
Some people have reported adverse reactions to the WET method precipitate or powder. This may be due to the Gilcrest precipitates which occur above pH 11.5. The DRY method removes the dangerous Gilcrest precipitates, so it results in safer material.
Hydrochloric acid. You can use muriatic acid (31% HCl) from a hardware store, but lab-grade HCl is less likely to be contaminated. Other acids can be used, but HCl will not harm the body if accidentally ingested in weak solutions and in small amounts. You might prefer to use distilled white vinegar instead of HCl. Although distilled white vinegar (acetic acid) is weaker than HCl, is it safer to work with.
Heavy plastic HDPE cottage cheese containers, 1 pint and 1 quart, to hold the coffee filters.
2. Across the bottom of the pint container, punch or drill several holes, 1/8" to 1/4" diameter, about 1/4" apart.
3. If the small container fits too tightly into the larger container, you may need to drill some air-pressure equalization holes around the outside of the large container near the level of the bottom of the small container. Otherwise the air pressure between the two containers will keep liquid from draining from the coffee filters. When you use this filter, place the cottage cheese containers in a stainless steel or glass container to catch any overflow. The lye water that you will be filtering may damage counter tops or cabinets if it contacts them.
4. The coffee filters should fit nicely into the smaller top cottage-cheese container.
These are some materials that produce a lot of precipitate from the DRY method:
First you need to prepare a dilute lye solution. Label an eyedropper bottle or squirt bottle "Lye-poison" so the bottle will not confused with something else. Work in a sink so that any spills will be contained. Lye gives off eye-stinging fumes when mixed with water. To avoid inhaling fumes, hold your breath and wear goggles while doing the following procedure.
Working over a sink, put 8 teaspoons of distilled water in a sturdy glass then stir in 1 teaspoon of lye. Stir until the lye is dissolved. Heat will be generated as the lye dissolves and the glass may get fairly hot. You may want to close your eyes to avoid eye-stinging fumes, taking a peek periodically.
Pour the lye solution into a labeled eyedropper bottle or squirt bottle.
If you are using pH paper, tear off several 1/4" pieces and put
them
on a piece of white paper on a plate (as illustrated above). Now
proceed as described below:
2. Add 1:4 lye solution to cover the dry material with a thin layer.
3. Stir in some distilled water to cover the powder and lye by 2 inches.
4. Bring to a boil (this is best done outdoors or in an exhaust hood). The pH should be at or slightly above 12. The lye brings the m-state elements into solution while leaving the Gilcrest precipitates as solids.
NOTE: If you start with sea salt, you can omit the boiling step with its noxious fumes, and simply let the solution sit for three days. Then go directly to Step 7. (Some other starting materials might also react without boiling).
5. If you are boiling the solution, replace water as needed to maintain sufficient reactant volume.
6. Boil for several hours -- the longer the better -- in a closed container. The container may be open if you add liquid as needed. Four hours should be sufficient for Etherium/Isis material.
7. Strain the slurry through 3 to 5 layers of coffee filters. You are removing the toxic elements (Gilcrest precipitate) that precipitate above pH 11.5.
Save the liquid that passes through the filters. Most m-state present will be in solution in the liquid.
8. While stirring the liquid, slowly add HCl or distilled white vinegar to bring the pH down to 8.5. A white precipitate forms which is partly m-state.
If you go too far, the pH will abruptly shift, and you will have to start over. If this happens you must add lye quickly and bring the pH back up to 12.
9. Let the precipitate settle overnight.
10. Using a large syringe (or a siphon), remove the liquid above the slurry.
11. Add distilled water to the precipitate (filling the jar), stir thoroughly, and let it settle again for at least 4 to 5 hours, preferably overnight.
12. Repeat steps 10 and 11 at least three times to thoroughly wash the precipitate. This removes most traces of lye and HCl (or vinegar).
You'll get a wet, white precipitate (slurry) containing m-state elements. Check that the pH is 9 or less before ingesting. Some of the precipitate may be milk of magnesia or calcium. If you wish, you can remove them using the precipitate purification procedures described above.
This method produces pure gold ORMUS. With this method, you must boil some lye solution for one to two weeks. This may spray some caustic solution around your working area. Please wear neoprene gloves, a PVC lab apron, and eye goggles when you use this method. Sources for this safety clothing are noted under LAB SUPPLIES near the end of this document.
Hydrochloric acid (HCl). You can use muriatic acid (31% HCl) from a hardware store, but lab-grade HCl is less likely to be contaminated. Instead of HCl, you might prefer to use distilled white vinegar (acetic acid). Distilled white vinegar is weaker than HCl but is safer to use.
A stainless steel pot or glass pot will work, but stainless steel and glass are attacked by NaOH. Glass is preferred over stainless steel.
A preferred container is a sealed Teflon® or HDPE bottle in a water bath in a crock pot. Please note that a Teflon® bottle is NOT the same as a Teflon® coated aluminum container. Never use aluminum or Teflon® coated aluminum containers and utensils because aluminum will react with acids like HCl and alkalis like lye, and will poison you.
If you use a sealed Teflon® or HDPE bottle in a water bath fill it only half full with your lye and gold then squeeze the bottle to eliminate most of the air above the liquid before you tighten the cap. This will allow the bottle to expand as the liquid is heated.
2. Boil the solution for two weeks in a CLOSED container. One week may be sufficient, but two weeks will likely have a higher yield. Add water as needed. CAUTION: Do not inhale the vapors!
3. Strain the solution using the coffee filter holder described above. Save any remaining gold for future use.
4. Add HCl or distilled white vinegar to bring the pH down to pH 8.5. An off-white precipitate will appear. Let it settle overnight.
5. Using a syringe, carefully suck out the liquid above the precipitate.
6. Add distilled water to the precipitate (filling the jar), stir thoroughly, and let it settle again for at least 4 to 5 hours, preferably overnight.
7. Repeat steps 5 and 6 at least three times to thoroughly wash
the
precipitate.
* Glass mason jars with wire-clamped glass lids and rubber gaskets
* Glass jars with plastic lids, or
* HDPE containers, which are stable in acid and alkali
Store m-state materials in the dark away from sunlight or ultraviolet light. Ultraviolet light seems to move some m-state materials toward a metallic state.
Because m-state materials are superconductors, they should be stored in glass or HDPE containers inside of steel containers and away from moving magnetic fields. Put the glass or HDPE container (containing m-state) inside a steel tin such as those used for Christmas cookies, gourmet popcorn or potato chips. If you intend to transport m-state materials, it is best to nest three or four steel containers, one inside the other with insulating material between, and place the glass or HDPE container inside the inmost steel container.
You may notice that your m-state materials have bubbles rising from them for a period of time after they are made. We believe that these bubbles are m-state gas escaping. Some people have reported that the m-state precipitate loses some of its effect as these bubbles leave. This off-gassing seems to be reduced if the m-state is stored between room temperature and body temperature in a magnetically shielded container. It is advisable not to refrigerate m-state materials. At least one researcher reports that refrigerated m-state materials are likely to move toward a warmer place.
Since bacteria and mold can easily grow in m-state precipitate, it is best to sterilize any material which you wish to store for long periods of time and to store it using water bath canning methods.
This supplier sells gold bullion, gold wire, gold shot, etc. They sell to individuals through the mail and will process small orders. Good prices. _________________________________________________
Strem Chemicals, Inc.
Dexter Industrial Park
7 Mulliken Way
Newburyport, MA 01950-4098
info@strem.com
http://www.strem.com
No minimum order.
(978) 462-3191
(800) 647-8736
Some sample products:
93-7915 Gold powder (99.95%) 500 mg $50. 2g
$160.
93-7913 Gold shot (99.95%) 500 mg $40. 2g $128.
Weiss Research Inc.
P.O. Box 720109
Houston, TX 77272
1-888-44-Weiss and fax: 281-879-9666 (24/7 fax)
http://www.weissresearch.com
They have a $175 pH meter (# PHM-150) with auto temperature
compensation
from 0 to 100 degrees C, and a pH meter with manual temperature
conversion
for $125.
McMaster-Carr Supply Co.
P.O. Box 4355
Chicago, IL 60680-4355
630-833-0300
fax 708-834-9427
http://www.mcmaster.com
McMaster-Carr Supply Co.
P.O. Box 54960
Los Angeles, CA 90054-0960
Sales & Customer Service: 562-692-5911
Fax: 562-695-2323 (24/7 fax)
Plastic syringe 50 cc with tapered tip 7510A665 pkg.
of
10 $18.57
Neoprene gloves 5278T3 (S,M,L,XL,XXL)
Neoprene Rubber gloves 5283T6 (M,L,XL) size needs to be specified.
Wide-range pH test paper 8707T11 $8.89
PVC apron 53445T75 $4.55
Safety goggles with a face shield 5422T12 $18.94.
Also available are a pocket pH meter for $59, pH solutions,
water-test kits, hot plates, plastic tubing, and much more.
No minimum order. They sell to anyone and take VISA.
Edmund Scientific Company
101 East Gloucester Pike
Barrington, NJ 08007-1380 USA
Customer service: 1-609-573-6260 9 AM to 5 PM M-F
Disposable plastic syringes are available at many veterinary and agricultural supply stores. Plastic infusion tips are available from the same source.
More sources can be found at:
http://monatomic.earth.com/database/lab-sources/
Sea salt to reconstitute sea water: Health-food store, oriental food store.
Target Glacial Rock Dust from Gaia Resources in Grand Forks, BC, Canada.
Get gold from a coin dealer.
Get gold dust by panning for it in a stream.
Gold dust might be available from Keene Engineering in Northridge,
CA.
See CHEMICAL SUPPLIERS earlier in this Appendix.
Also check out a gold mining supply store.
In California try http://www.treasurenet.com/calgold/prospect.html
The gold should be at least 99.99% pure gold.
Golden Nectar (trace minerals). $40/gallon.
http://bulksales.com/index.htm
This may be at health food stores.
Trace Minerals Inland Sea Water ($7 for 8 ounces) at your local
health
food store.
Trace Minerals Research, P.O. Box 429, Roy,
UT 84076.
http://www.traceminerals.com
http://bulksales.com
Etherium white powder gold: http://www.etheriumgold.com/
phone 530-272-1511
Isis white powder gold: http://onlinehealth.web2010.com/isis.html